Team develops human organ-like cell culture models to speed chemical testing, assess safety
Editors: Contact Elizabeth Sharpe (206.686.6737) to interview UW faculty quoted in the release below.

Tens of thousands of chemicals are currently in use, with more introduced every year. Scientists, however, have discerned the toxicity to human health for only a fraction of these, because the traditional method of testing is time-and cost-prohibitive. Thus, the need for high-capacity in vitro systems to screen chemicals for their potential health impacts is significant.
The U.S. Environmental Protection Agency today announced that it will provide $6 million in seed funding for a Predictive Toxicology Center at the University of Washington, enabling researchers to develop more accurate in vitro models – organ-mimicking cell cultures – to test chemicals for their potential risk to humans and to help accelerate the evaluation of large numbers of chemicals.
“We are proud to invest in building a new, three dimensional way of looking at how toxic chemicals affect people and all living things,” said Michelle Pirzadeh, EPA’s deputy regional administrator in Seattle.

The center's research is important to establish novel methods that reflect the complexity of biological systems, "and allow us to evaluate the large number of new chemicals for potential health impacts before they are put on the market,” said Elaine Faustman. She and Terrance Kavanagh, both UW professors of environmental and occupational health sciences, will co-direct the new center. It is part of the Institute for Risk Analysis and Risk Communication.
Researchers from the Schools of Public Health, Pharmacy, and Medicine will collaborate to develop these three-dimensional cell cultures for the kidney, liver, lung and testis to better model how a person would respond to a chemical exposure.
The cells can metabolize or excrete a chemical in response to a toxic chemical exposure, Kavanagh explained. Replicating the flow of blood, polarity (the pull between opposite sides of a cell), and the various enzymes and proteins essential to organ function are critical to the testing of environmental chemical interactions with human biology. These in vitro systems can help reduce the need for animal models while better replicating and more faithfully mimicking what happens in the human body, including differences in genetics and susceptibility, Kavanaugh said.
A hallmark of the center’s research will be microfluidic chip technology developed by Nortis, a biotech founded by former UW faculty. Less than half the size of an index card, the microfluidic chip offers a platform for cells to be seeded onto the device, which then serves as a unique growing environment and can be connected with the other devices for each organ system, mimicking human circulation.
“In order to qualify these systems, it’s good to work with tested compounds, those we already know quite a bit about,” said Kavanagh. He will co-manage the liver-testing model with David Eaton, UW professor of environmental and occupational health sciences.

Kavanagh leads a team at the UW that has been standardizing techniques and analytical tools to assess and predict the toxicity and environmental impact of engineered nanomaterials.
Testing metals and metal-based nanomaterials will be a key focus of the new center. “There’s been a lot of interest in the biomedical field to use nanomaterials as therapeutics and bioimaging agents,” he said. However, the potential for environmental and occupational nanomaterial exposures in the general population and their unintentional impacts is less well studied.
William Altemeier, UW associate professor of pulmonary and critical care medicine, will lead the lung cell culture’s development. “A key route of exposure to nanomaterials is potentially via inhalation thus developing a lung system is especially relevant,” he said.
The three-dimensional models will be able to track exposure to chemicals for an extended period of time, which is significant because it allows for the systems to mimic low-dose, chronic exposures, most likely found in human populations. For example, some chemical exposures are associated with negative affects on reproduction, even at low doses. Faustman will lead refinement of a three-dimensional in vitro model to test adverse reactions in testicular development.
Edward Kelly, UW associate professor of pharmaceutics in the School of Pharmacy, will manage the project on the kidney cell culture. Preliminary testing shows that the device works as a kidney would, expressing critical proteins and demonstrating the polarity characterized by the type of cells found in this organ. “The cells attach, proliferate, and form the same shape they would in a human body and thus replicate many of a kidney’s normal functions,” he said.
When these cells are exposed to a heavy metal, Kelly explained, a “toxicant signature” or biomarker of tissue injury is evident, such as an increased level of protein molecules expressed.
“These systems are being used in our lab studies to test drugs with known adverse effects on the kidneys, including antibiotics, chemotherapies, and immunosuppressants,” he said.
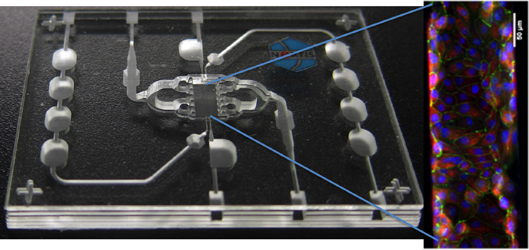
Kelly and Eaton are involved in another research collaboration at the UW to get organ models like the intestines, liver and kidney to work together. That work is funded by the National Institutes of Health. The new EPA-funded Predictive Toxicology Center will build on this work and apply these organ models to assess environmentally relevant chemicals.
After validating these three-dimensional cell cultures using known toxicants, the Predictive Toxicology Center researchers will investigate biomarkers of cell injury or altered function that indicate an adverse outcome from an exposure to those chemicals for which insufficient data exists.
They will then use modeling techniques to assess and predict human health risks, linking laboratory data with human exposure pathways.
The center will use computational models to identify and characterize adverse outcomes from environmental chemicals, focusing on the importance of time of exposure, genetics and oxidative stress as mediators of toxicity. Such an approach will allow for connecting in vitro model results with human physiological changes and better predict a chemical’s potential for human health effects.